Topic 2 Bonding, structure and properties of matter

Home » AQA GCSE CHEMISTRY » Topic 2 Bonding, structure and properties of matter

This topic uncovers the secrets of how atoms link together, building the structure of every substance in the universe and giving them their unique properties, from the hardness of a diamond to the fluidity of water.
A chemical bond is a force of attraction that holds atoms together. Atoms form these bonds to achieve a more stable electron arrangement, which usually means having a full outer shell of electrons.
For most atoms, a full outer shell means having eight electrons (the ‘octet rule’). The main exceptions are hydrogen and helium, which are stable with just two electrons. This drive to achieve a full outer shell is the reason why chemical reactions happen.
Exam Tip: When asked to define a chemical bond, state that it’s a force holding atoms together, allowing them to achieve a stable, full outer shell of electrons.
There are three main types of strong chemical bonds you need to know: ionic, covalent, and metallic.
What is it? An ionic bond forms between a metal and a non-metal.
How does it work? The metal atom loses electrons to become a positively charged ion, and the non-metal atom gains those electrons to become a negatively charged ion. The bond is the strong electrostatic force of attractionbetween these oppositely charged ions.
What is it? A covalent bond forms between two non-metal atoms.
How does it work? The atoms share one or more pairs of electrons. This sharing allows each atom to achieve a full outer shell. This can form simple molecules (like H₂O and CH₄) or giant covalent structures (like diamond).
What is it? Metallic bonding is found in metals and alloys.
How does it work? The metal atoms lose their outer shell electrons, which become delocalised. This creates a lattice of positive metal ions surrounded by a “sea” of free-moving electrons. The bond is the strong attraction between the positive ions and the delocalised electrons.
Ionic bonding happens between a metal and a non-metal. It involves the transfer of electrons from the metal atom to the non-metal atom. This transfer allows both atoms to achieve a stable, full outer shell.
The process happens in two key steps:
Electron Transfer: The metal atom loses one or more electrons from its outer shell, becoming a positively charged ion (cation). The non-metal atom gains these electrons to complete its outer shell, becoming a negatively charged ion (anion).
Electrostatic Attraction: The resulting oppositely charged ions (the positive metal ion and the negative non-metal ion) are strongly attracted to each other. This powerful force of attraction is the ionic bond.
Exam Tip: A complete answer on ionic bonding must describe both the transfer of electrons and the resulting electrostatic attraction between the oppositely charged ions.
Let’s look at how sodium (a Group 1 metal) reacts with chlorine (a Group 7 non-metal).
A sodium atom has one electron in its outer shell (electron configuration 2.8.1). It loses this electron to achieve a stable configuration of 2.8, forming a sodium ion with a +1 charge (Na⁺). Na (2.8.1) ⟶ Na⁺ (2.8) + e⁻
A chlorine atom has seven electrons in its outer shell (electron configuration 2.8.7). It gains one electron to achieve a stable configuration of 2.8.8, forming a chloride ion with a -1 charge (Cl⁻). Cl (2.8.7) + e⁻ ⟶ Cl⁻ (2.8.8)
The positive sodium ion (Na⁺) and the negative chloride ion (Cl⁻) are then held together by a strong electrostatic attraction, forming the compound sodium chloride.
Ionic compounds are formed from the strong electrostatic attraction between positive and negative ions. This attraction results in a highly ordered, three-dimensional structure.
An ionic compound is not made of simple molecules. Instead, it forms a giant ionic lattice, which is a regular, repeating arrangement of cations (positive ions) and anions (negative ions). Each ion is strongly attracted to all the surrounding ions of the opposite charge, creating a very strong structure.
We can represent ionic compounds in several ways, but it’s important to understand the strengths and weaknesses of each model.
This model is useful for showing how the electrons are transferred from the metal atom to the non-metal atom to form ions.
Limitation: It is only a 2D representation and doesn’t show the vast, repeating structure of the lattice.
This is a simplified diagram showing the alternating positive and negative ions in a grid format.
Limitation: It is flat and fails to show the true three-dimensional arrangement of the ions.
This 3D model shows the regular arrangement of ions (as spheres) and the ionic bonds between them (as sticks). It gives a better sense of the lattice structure.
Limitation: The model can be misleading as it exaggerates the distances between the ions; in reality, they are packed closely together. It also incorrectly suggests that the forces of attraction only exist in the direction of the “sticks.”
The empirical formula gives the simplest whole-number ratio of ions in the compound. You can determine this by counting the ratio of cations to anions in a model.
Example: Magnesium Chloride If you look at the structure of magnesium chloride, for every one magnesium ion (Mg²⁺), there are two chloride ions (Cl⁻).
Ratio of ions: 1 Mg²⁺ : 2 Cl⁻
Therefore, the empirical formula is MgCl₂.
Covalent bonding is a fundamental way that atoms join together, occurring when two non-metal atoms share pairs of electrons. The goal of this sharing is for each atom to achieve a stable, full outer shell.
This type of bonding is responsible for creating two very different kinds of substances: small, individual molecules and vast, strong structures.
A covalent bond is formed when two atoms each contribute one or more electrons to form a shared pair. This shared pair of electrons effectively belongs to both atoms, helping each to fill its outer shell. For example, in a hydrogen molecule (H₂), each hydrogen atom shares its single electron with the other, allowing both to have a full outer shell of two electrons.
These substances are made of individual, discrete molecules. Think of water (H₂O) or carbon dioxide (CO₂).
Structure: Within each molecule, the atoms are held together by very strong covalent bonds. However, the forces of attraction between the molecules (intermolecular forces) are very weak.
Properties:
Low melting and boiling points: Only a small amount of energy is needed to overcome the weak intermolecular forces, so these substances are often liquids or gases at room temperature.
Poor electrical conductors: The molecules have no overall charge and there are no free-moving electrons or ions to carry a current.
Examples: Hydrogen (H₂), Chlorine (Cl₂), Water (H₂O), Methane (CH₄).
Exam Tip: When discussing simple molecules, it’s crucial to distinguish between the strong covalent bondswithin the molecules and the weak intermolecular forces between them. It’s these weak forces that are broken when the substance melts or boils.
Metallic bonding is the type of bonding found in metals and alloys. It involves metal atoms losing their outer shell electrons to form a regular lattice of positive ions surrounded by a “sea” of delocalised electrons. This unique structure is responsible for the characteristic properties of metals.
The model for metallic bonding can be broken down into two main components:
A Lattice of Positive Ions: The metal atoms lose their valence (outer shell) electrons and become positively charged ions (cations). These ions pack together in a regular, repeating, three-dimensional arrangement called a lattice.
A “Sea” of Delocalised Electrons: The outer electrons are no longer associated with any single atom. Instead, they become delocalised, meaning they are free to move throughout the entire metal structure.
The metallic bond itself is the strong electrostatic force of attraction between the positive metal ions and the negatively charged sea of delocalised electrons.
This model perfectly explains why metals behave the way they do.
| Property | Explanation |
| Good Electrical Conductivity | The delocalised electrons are free to move and carry an electrical current when a voltage is applied. |
| Good Thermal Conductivity | The free-moving electrons can also transfer kinetic (heat) energy quickly throughout the lattice. |
| Malleable and Ductile | The layers of positive ions can slide over one another without breaking the metallic bond. The sea of electrons flows around them, maintaining the attraction and holding the structure together. This allows metals to be hammered into shape (malleable) or drawn into wires (ductile). |
| High Melting & Boiling Points | There is a strong electrostatic attraction between the positive ions and the delocalised electrons. A large amount of energy is required to overcome these strong forces and break down the lattice. |
Exam Tip: When explaining the properties of metals, a good answer will always mention both the positive ions arranged in a lattice and the mobile (or delocalised) electrons. Be prepared to use this model to explain at least two different properties.
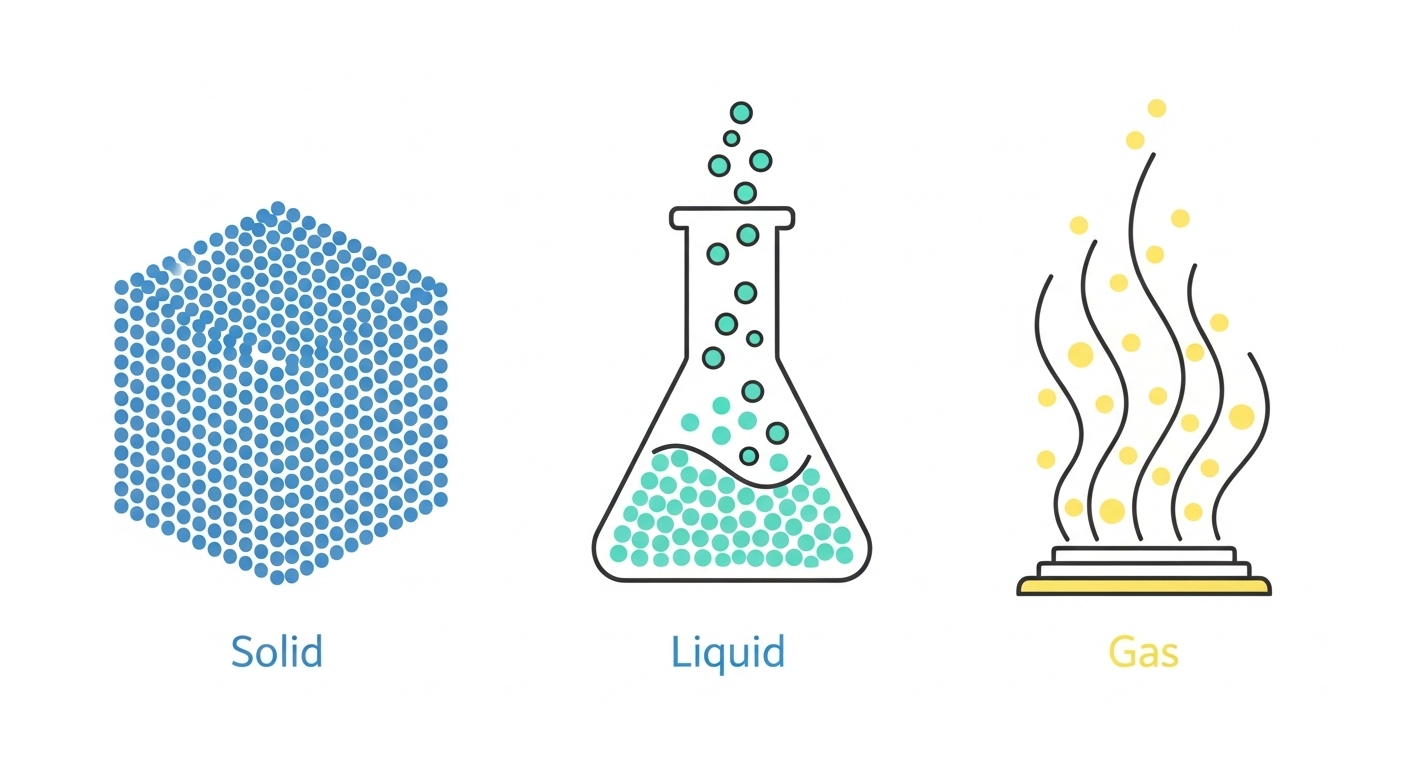
Matter can exist in three main states: solid, liquid, and gas. The state of a substance at a given temperature and pressure depends on the energy of its particles and the strength of the forces between them.
Particle Arrangement: In a solid, particles are packed tightly together in a fixed, regular pattern called a lattice.
Forces: There are strong forces of attraction holding the particles in place.
Movement: The particles don’t move from their positions. Instead, they vibrate on the spot.
Properties:
Have a fixed shape and volume.
Cannot be compressed.
Particle Arrangement: In a liquid, the particles are still close together but are arranged randomly.
Forces: There are forces of attraction between the particles, but they are weaker than in a solid.
Movement: The particles can move and slide past one another, allowing the liquid to flow.
Properties:
Have a fixed volume but take the shape of their container.
Are very difficult to compress.
Particle Arrangement: In a gas, the particles are very far apart and are arranged randomly.
Forces: The forces of attraction between particles are very weak.
Movement: The particles move quickly and randomly in all directions, and with more energy than in a liquid.
Properties:
Have no fixed shape or volume; they will expand to fill any container.
Are easily compressed.
Exam Tip: When explaining changes of state (like melting or boiling), you must talk about energy. For example, to melt a solid, energy is supplied to the particles, which makes them vibrate more. This energy is used to overcome the forces of attraction, allowing the particles to move freely as a liquid.
In chemical equations, we use state symbols to show the physical state of each substance involved in a reaction. For AQA GCSE, you need to know and use four specific symbols.
| Symbol | State | Meaning & Context |
| (s) | Solid | The substance is a solid at the reaction temperature. |
| (l) | Liquid | The substance is a pure liquid. |
| (g) | Gas | The substance is a gas. |
| (aq) | Aqueous | The substance is dissolved in water to form a solution. |
These symbols are written immediately after the chemical formula. They are important because they clarify how a reaction is taking place and what you would observe.
This is where two aqueous solutions react to form an insoluble solid, called a precipitate.
AgNO₃(aq) + NaCl(aq) ⟶ AgCl(s) + NaNO₃(aq) Here, silver chloride (AgCl) is the solid precipitate formed.
When an acid and an alkali react, a salt is formed in solution and water is produced as a pure liquid.
HCl(aq) + NaOH(aq) ⟶ NaCl(aq) + H₂O(l)
Notice that water is shown as (l), not (aq), because it is the solvent.
When a solid is heated and breaks down, it can produce other solids and release a gas. CaCO₃(s) ⟶ CaO(s) + CO₂(g)
Here, solid calcium carbonate breaks down into solid calcium oxide and carbon dioxide gas.
Exam Tip: State symbols are particularly important in questions about precipitation. Always remember to identify the insoluble solid product and give it the (s) symbol, while the dissolved reactants and other products are labelled (aq).
The structure of ionic compounds, a giant lattice held together by strong forces, gives them a distinct set of physical properties. You need to be able to describe these properties and explain them using your knowledge of the ionic lattice.
Ionic compounds have very high melting and boiling points. For example, sodium chloride (NaCl) melts at 801 °C and boils at 1413 °C.
Explanation: The giant ionic lattice is held together by strong electrostatic forces of attraction between the oppositely charged ions. A large amount of energy is needed to overcome these forces and break down the lattice, allowing the ions to move freely as a liquid or gas.
Whether an ionic compound can conduct electricity depends entirely on its state.
| State | Conducts Electricity? | Explanation |
| Solid | No | In the solid lattice, the ions are fixed in place and cannot move to carry an electrical charge. |
| Molten (Liquid) | Yes | When melted, the ions are free to move and can act as mobile charge carriers. |
| Aqueous (Dissolved) | Yes | When dissolved in water, the ions separate and are free to move throughout the solution, allowing them to carry a charge. |
Many, but not all, ionic compounds can dissolve in water. When they dissolve, the water molecules surround the individual ions, breaking down the lattice structure and allowing the ions to move freely within the solution.
Exam Tip: When answering questions about the properties of ionic compounds, you must always link your explanation back to the structure. Key phrases to use are “strong electrostatic forces” when talking about melting points, and “ions are free to move” (or not free to move) when discussing electrical conductivity.
Substances made of small molecules (also called simple molecular substances) consist of non-metal atoms joined by strong covalent bonds. Their physical properties are determined not by the strong bonds inside the molecules, but by the weak forces between the molecules.
Simple molecular substances are made of a small, fixed number of atoms, such as water (H₂O), carbon dioxide (CO₂), and methane (CH₄). Within each molecule, the atoms are held together by very strong covalent bonds. However, these individual molecules are attracted to each other by very weak intermolecular forces.
Simple molecular substances have low melting and boiling points. This is because only a small amount of energy is needed to overcome the weak intermolecular forces between the molecules. The strong covalent bonds within the molecules do not break. As a result, many of these substances are gases or liquids at room temperature.
Simple molecular substances do not conduct electricity in any state. This is because the molecules are neutral and there are no free-moving electrons or ions to carry an electrical charge.
Exam tip: When explaining the low melting and boiling points of simple molecular substances, you must refer to the need to overcome weak intermolecular forces, not the strong covalent bonds.
Polymers are very large molecules, often called macromolecules, built from many smaller, repeating units joined together. These repeating units are called monomers. The properties of polymers are unique, differing from both simple molecules and giant covalent structures.
Polymers are formed when thousands of monomer molecules join together in a long chain. This process is called polymerisation.
Bonds within the chain: The atoms along the polymer chain are joined by very strong covalent bonds.
Forces between chains: The individual polymer chains are held together by intermolecular forces. These forces are stronger than those found between simple molecules (which is why polymers are solid at room temperature), but they are weaker than the covalent or ionic bonds found in giant structures.
The properties of a polymer depend on the strong bonds within its chains and the intermolecular forces between them.
| Property | Explanation |
| Solid at room temperature | The intermolecular forces between the long polymer chains are strong enough to hold them together in a solid state. |
| Melting Point | Polymers have higher melting points than simple molecular substances because more energy is needed to overcome the stronger intermolecular forces between the longer chains. However, they usually melt at lower temperatures than giant ionic or covalent structures. |
| Flexibility | The properties can vary. Some polymers have chains that are tangled but can slide over each other, making them flexible. Others have cross-links between the chains, making them more rigid and strong. |
This is a common polymer made from ethene monomers. The double bond in each ethene molecule breaks to form a long saturated chain. It is used to make plastic bags, bottles, and films.
This polymer is made from propene monomers. It is generally stronger and more rigid than poly(ethene) and is used to make ropes, crates, and carpets.
Exam Tip: Do not confuse the strong covalent bonds within the polymer chains with the weaker intermolecular forces between them. It is the intermolecular forces that are overcome when a polymer melts.
Giant covalent structures are substances where huge numbers of non-metal atoms are joined to each other by a continuous network of strong covalent bonds. Their properties are very different from those of simple molecules or polymers because there are no separate molecules and no weak intermolecular forces.
In a giant covalent structure, every atom is linked to its neighbours by strong covalent bonds. This creates a single, massive three-dimensional lattice. Key examples that you need to know are diamond, graphite, and silicon dioxide (SiO₂).
| Property | Explanation |
| Very High Melting & Boiling Points | A very large amount of energy is required to break the millions of strong covalent bonds throughout the entire structure. |
| Hardness | Most giant covalent structures are extremely hard and rigid because the atoms are held in a fixed, tightly bonded network. |
| Solubility | They are insoluble in water as the strong covalent bonds cannot be broken by the water molecules. |
| Electrical Conductivity | This varies. It depends entirely on whether the structure has delocalised electrons that are free to move. |
In diamond, each carbon atom forms four strong covalent bonds with four other carbon atoms, creating a rigid tetrahedral arrangement.
Properties: It is the hardest known natural substance, has an extremely high melting point, and does not conduct electricity because all its outer-shell electrons are locked in covalent bonds and are not free to move.
In graphite, each carbon atom forms three strong covalent bonds with three other carbons, creating layers of interconnected hexagonal rings.
Properties: The layers are held together by weak forces, allowing them to slide over each other, which makes graphite soft and slippery. Each carbon atom has one delocalised electron that is free to move along the layers, allowing graphite to conduct electricity.
Also known as silica (the main component of sand), this has a structure similar to diamond. Each silicon atom is bonded to four oxygen atoms, and each oxygen atom is bonded to two silicon atoms.
Properties: It is very hard, has a high melting point, and does not conduct electricity as there are no free-moving electrons.
Exam Tip: When explaining the properties of a giant covalent structure, you must always link your answer back to its specific structure and bonding. Simply naming the substance is not enough. For example, “Diamond is hard because each carbon is held in a rigid lattice by four strong covalent bonds.”
Metals have a giant structure with strong metallic bonding. Their outer-shell electrons are delocalised and move throughout the lattice, which explains many of their bulk properties.
Structure and bonding
Metallic lattice: positive metal ions in regular layers, surrounded by a sea of delocalised electrons. The strong electrostatic attraction between ions and delocalised electrons gives a robust structure with characteristic properties.
Properties and explanations
| Property | What you observe | Explanation using structure and bonding |
|---|---|---|
| High melting point and boiling point | Metals stay solid to high temperatures | Strong metallic bonds require lots of energy to overcome. |
| Malleable and ductile (can be bent and drawn into wires) | Pure metals are easily shaped | Atoms are arranged in layers that can slide over each other. |
| Good electrical conductor | Metals allow current to pass | Delocalised electrons carry charge through the structure. |
| Good thermal conductor | Metals transfer heat quickly | Energy is transferred by the movement of delocalised electrons. |
Why alloys are harder than pure metals
Most everyday metals are actually alloys.
| Alloy | Composition | Key property or note | Typical use |
|---|---|---|---|
| Bronze | Copper and tin | Tough and corrosion resistant | Medals, statues, bearings |
| Brass | Copper and zinc | Harder than copper, easily shaped | Instruments, fittings |
| Gold alloys | Gold with silver, copper and zinc | Purity measured in carats (24 carat is pure, 18 carat is 75 percent) | Jewellery |
| High-carbon steel | Iron with more carbon | Very strong, brittle | Cutting tools |
| Low-carbon steel | Iron with little carbon | Softer, easily shaped | Car bodies, panels |
| Stainless steel | Iron with chromium and nickel | Hard, corrosion resistant | Cutlery, chemical plant |
| Aluminium alloys | Aluminium with other metals | Low density | Aircraft, lightweight structures |
Exam tip: When a question asks why a metal or alloy has a property, link it to structure and bonding. For example, explain hardness in terms of layer distortion in alloys or conductivity in terms of delocalised electrons.
Metals are excellent electrical and thermal conductors. The reason is their metallic bonding: positive metal ions in a lattice surrounded by delocalised electrons that are free to move through the structure.
These are material or sample factors you can reference in explanations or calculations in science questions.
Exam tip: When you explain conduction, always mention delocalised electrons and link to structure and bonding. For thermal conduction, state that electrons transfer energy and that lattice vibrations also play a role. Avoid saying current flows because “metals are shiny” or “metals are strong” as these are not the causes.
Diamond is an allotrope of carbon, meaning it’s a form of pure carbon with a distinct structure. It is a classic example of a giant covalent structure.
The unique properties of diamond all stem from its internal structure and bonding.
Atoms: It is made up only of carbon atoms.
Bonding: Each carbon atom forms four strong covalent bonds with four other carbon atoms.
Arrangement: These bonds are arranged in a rigid tetrahedral shape, which repeats to form a vast, continuous 3D lattice.
Electrons: All four of carbon’s outer shell electrons are used to form these bonds. This means there are no delocalised (free) electrons.
You need to be able to explain each of diamond’s properties by linking them directly to its structure and bonding.
| Property | Observation & Explanation |
| Very High Melting Point | A very large amount of energy is needed to overcome the millions of strong covalent bonds throughout the giant lattice. This is why diamond remains solid at extremely high temperatures. |
| Extremely Hard | Diamond is one of the hardest known natural substances. This is because each atom is held tightly in place by four strong covalent bonds in a rigid 3D network, preventing the atoms from being moved or scratched. |
| Does Not Conduct Electricity | Diamond is an electrical insulator. This is because there are no delocalised electrons or ions that are free to move and carry an electrical charge. |
| Insoluble | Diamond does not dissolve in any solvent. The strong covalent bonds holding the atoms together are too powerful to be broken by solvent molecules. |
The unique properties of diamond make it ideal for specific applications.
Cutting tools and drill tips: Its extreme hardness allows it to cut through other tough materials like rock and metal.
Jewellery: Its rigid lattice structure and the way it interacts with light give it a brilliant sparkle, while its hardnessmakes it durable and resistant to scratches.
Exam Tip: When explaining the properties of diamond, you must use specific language. For its high melting point and hardness, refer to the “many strong covalent bonds in a giant lattice“. For its electrical conductivity, you must state that it has “no delocalised electrons“.
Graphite is another allotrope of carbon, but its properties are dramatically different from diamond. It is a giant covalent structure where atoms are arranged in a unique layered pattern.
The key to graphite’s properties is its layered structure.
Atoms: It is made up only of carbon atoms.
Bonding: Each carbon atom forms three strong covalent bonds with its neighbours, creating interlocking hexagonal rings.
Arrangement: These rings form vast, flat sheets or layers.
Between the Layers: The layers themselves are not held by covalent bonds. Instead, there are only weak forces of attraction between them.
Electrons: Since each carbon only uses three of its four outer electrons for bonding, there is one delocalised (free) electron per atom. These electrons are free to move along the layers.
Graphite is a good electrical conductor (for a non-metal). The delocalised electrons are free to move along the layers and carry a charge. However, it does not conduct well in a direction perpendicular to the layers, as the electrons cannot easily jump between them.
Graphite feels greasy and is used as a dry lubricant. This is because the weak forces between the layers are easily overcome, allowing the layers to slide over one another. When you write with a pencil, you are simply sliding layers of graphite onto the paper.
Like diamond, graphite has a very high melting point. Although the forces between the layers are weak, the bonds withinthe layers are strong covalent bonds. A vast amount of energy is needed to break these bonds and break down the entire structure.
Graphite’s unique combination of properties makes it useful for several applications.
Pencils and Lubricants: Its soft and slippery nature allows layers to slide off easily.
Electrodes: It is used for electrodes in electrolysis and in some batteries because it conducts electricity and has a high melting point, allowing it to withstand high temperatures.
Exam Tip: Be very specific in your explanations. For conductivity, you must mention the delocalised electrons that can move along the layers. For why it’s soft, you must state that the weak forces between layers allow them to slide. Never say that the covalent bonds are weak – they are very strong!
Graphene and fullerenes are fascinating allotropes of carbon. Like graphite, each carbon atom in these structures forms three covalent bonds, which means there is one delocalised electron per atom, leading to some extraordinary properties.
Graphene is a single, two-dimensional layer of graphite. It is just one atom thick and is arranged in a flat, hexagonal lattice.
The structure consists of carbon atoms joined by strong covalent bonds.
Each carbon atom forms three bonds, leaving one delocalised electron per atom that is free to move across the entire sheet.
| Property | Observation & Explanation |
| Excellent Electrical Conductor | The delocalised electrons are highly mobile and move freely across the sheet, allowing graphene to carry a current with very little resistance. |
| Very Strong and Light | Despite being incredibly light, the network of strong covalent bonds makes graphene one of the strongest materials ever tested. |
| High Thermal Conductivity | It is an excellent thermal conductor, as vibrations and mobile electrons transfer heat energy efficiently. |
| Transparent and Flexible | As a single layer of atoms, it is almost transparent and can be bent and stretched without breaking. |
Electronics: Its thin, flexible, and conductive nature makes it ideal for touchscreens, sensors, and high-speed electronic devices.
Composites: It can be added to other materials to increase their strength without adding significant weight.
Fullerenes are molecules of carbon where the atoms are arranged in hollow shapes, often containing rings of hexagons and pentagons. They include spherical molecules like Buckminsterfullerene (C₆₀) and cylindrical forms like carbon nanotubes.
Structure: This molecule consists of 60 carbon atoms arranged in a hollow sphere, resembling a football.
Bonding: Each carbon atom forms three covalent bonds. The delocalised electrons are spread over the surface of the molecule.
Properties: As a solid, C₆₀ forms a simple molecular structure. The individual C₆₀ molecules are held together by weak intermolecular forces. This means it is soft and has a much lower melting point than giant covalent structures.
Uses: It has potential uses in lubricants (as the spheres can roll), drug delivery systems (by trapping molecules inside), and as catalysts.
Structure: A carbon nanotube can be imagined as a sheet of graphene that has been rolled into a seamless cylinder.
Bonding: They have a very high tensile strength due to the strong covalent bonds throughout the tube. Like graphene, they have delocalised electrons that can move along the tube’s length.
Properties: They conduct electricity and heat very well and have a very large surface area to volume ratio.
Uses: They are used to reinforce composite materials to make them strong yet lightweight (e.g., in tennis rackets or bicycle frames) and have potential applications in nanoelectronics and catalysts.
Exam tip: Always link a property to structure and bonding. For C₆₀, explain its softness and low melting point by referring to the weak intermolecular forces between the molecules. For graphene and nanotubes, explain their strength by mentioning the strong covalent bonds and their conductivity by referring to the delocalised electrons.
The properties of a substance can change dramatically depending on the size of its particles. This section focuses on how particle size, particularly at the nanoscale, affects a material’s behaviour.
For your exams, you need to know three key size ranges for particles. These are typically measured in nanometres (nm).
Nanoparticles: Have a diameter from 1 nm to 100 nm. (A nanometre is one-billionth of a metre: 1 nm = 1 × 10⁻⁹ m)
Fine particles (PM₂.₅): Have a diameter from 100 nm to 2,500 nm.
Coarse particles (PM₁₀): Have a diameter from 2,500 nm to 10,000 nm.
The most important concept linking size to properties is the surface area to volume ratio (SA:V).
As particles get smaller, their surface area to volume ratio gets larger.
Imagine a single large cube. Now, imagine cutting that same large cube into many smaller cubes. The total volume remains the same, but the total surface area of all the smaller cubes is now much greater. This means a far greater proportion of the substance is on the surface.
A high SA:V ratio is important because it means:
Reactions can happen faster as there is more surface available for particles to collide and react.
Materials can be more effective catalysts.
Smaller quantities of a material are needed to achieve the desired effect, which can reduce costs.
Nanoparticles have the same atoms and bonding as their larger-scale counterparts, but their extremely high SA:V ratio gives them different physical and chemical properties.
Catalysts: Their huge surface area makes industrial reactions more efficient.
Sun Creams: Nanoparticles of titanium dioxide (TiO₂) or zinc oxide (ZnO) are very effective at blocking UV light but are so small that they appear transparent on the skin, unlike traditional white sunblock.
Antibacterial Coatings: Silver nanoparticles are used on surgical instruments and in plasters. Their high SA:V allows them to release silver ions which are toxic to bacteria.
Self-Cleaning Surfaces: Some coatings use nanoparticles that break down dirt when exposed to sunlight.
Drug Delivery: Nanoparticles can be used to carry drugs to specific target cells within the body.
While nanoparticles offer many benefits, there are also potential risks that are not yet fully understood.
Toxicity: Due to their small size, they may be able to enter the body through the skin, be inhaled, or be ingested.
Cellular Damage: It is possible that nanoparticles could pass into cells and cause damage. Extensive research is needed to assess these long-term health risks.
Environmental Impact: There are concerns that nanoparticles could accumulate in the environment and harm ecosystems.
Exam Tip: You must be able to recall the three size ranges. When explaining the unique properties or uses of nanoparticles, always link your answer back to their high surface area to volume ratio. For any given use, be prepared to name the material, its application, and the specific property that makes it suitable.
Nanoparticles are incredibly small particles, typically with a diameter ranging from 1 nm to 100 nm. To put that into perspective, one nanometre (nm) is a billionth of a metre (1 x 10⁻⁹ m). At this tiny scale, materials can exhibit properties that are very different from the same substance in bulk form.
The unique behaviour of nanoparticles is almost entirely due to their huge surface area to volume ratio (SA:V).
As a particle gets smaller, a greater proportion of its atoms are on the surface compared to its volume. Imagine a large cube of sugar. If you crush it into thousands of tiny grains, the total volume of sugar is the same, but the total surface area of all the grains is now enormous.
This high SA:V ratio means that nanoparticles are often much more reactive than the same mass of the material in bulk form.
Their high surface area to volume ratio gives nanoparticles several key properties:
High Reactivity: With more atoms at the surface, reactions can occur much more quickly.
Enhanced Catalytic Activity: They make excellent catalysts because they provide a very large surface area for reactions to take place on.
Unusual Optical Properties: Their small size can affect how they interact with light, meaning they can appear transparent whereas the bulk material might be opaque.
The unique properties of nanoparticles have led to their use in many new technologies and products.
Sun Creams: Nanoparticles of titanium dioxide and zinc oxide are used in sun creams. They are highly effective at blocking harmful UV light, but because the particles are smaller than the wavelength of visible light, they appear transparent on the skin, avoiding the white residue of older sunblocks.
Antibacterial Applications: Silver nanoparticles have powerful antibacterial properties. Their high SA:V allows them to release silver ions that can kill bacteria. They are used in wound dressings, deodorants, and coatings for appliances like refrigerators.
Catalysts: Their massive surface area makes them extremely efficient catalysts in industrial processes, meaning less of the (often expensive) material is needed.
Self-Cleaning Surfaces: Some glass and textiles are coated with nanoparticles that use energy from sunlight to break down dirt.
Medicine: Scientists are developing ways to use nanoparticles to deliver drugs directly to targeted cells, like cancer cells, within the body.
While nanoparticles have many benefits, their small size also raises potential health and environmental concerns.
Health Risks: Because they are so small, nanoparticles could potentially be inhaled, absorbed through the skin, or ingested. It is not yet fully understood what the long-term effects of this might be on the human body.
Environmental Impact: There are concerns that nanoparticles could accumulate in the soil or water, potentially harming ecosystems.
Extensive research and careful testing are required to fully understand and manage these risks.
Exam Tip: When a question asks why nanoparticles are useful for a specific application, your answer must be linked to their high surface area to volume ratio. For example, “Silver nanoparticles are used in antibacterial socks because their high surface area to volume ratio allows them to release a steady stream of silver ions that can kill bacteria.”